Unique type of chemical bond in enzyme bilirubin oxidase is a key for development of biotechnological applications
Fri Oct 04 00:00:00 CEST 2019
Sat May 02 21:45:38 CEST 2020 | Sat May 02 21:45:38 CEST 2020 - Sat May 02 21:45:38 CEST 2020
Increased level of bilirubin, product of red blood cells metabolism, in blood can be symptomatic for liver diseases, such as cirrhosis or hepatitis, or for bile duct diseases.
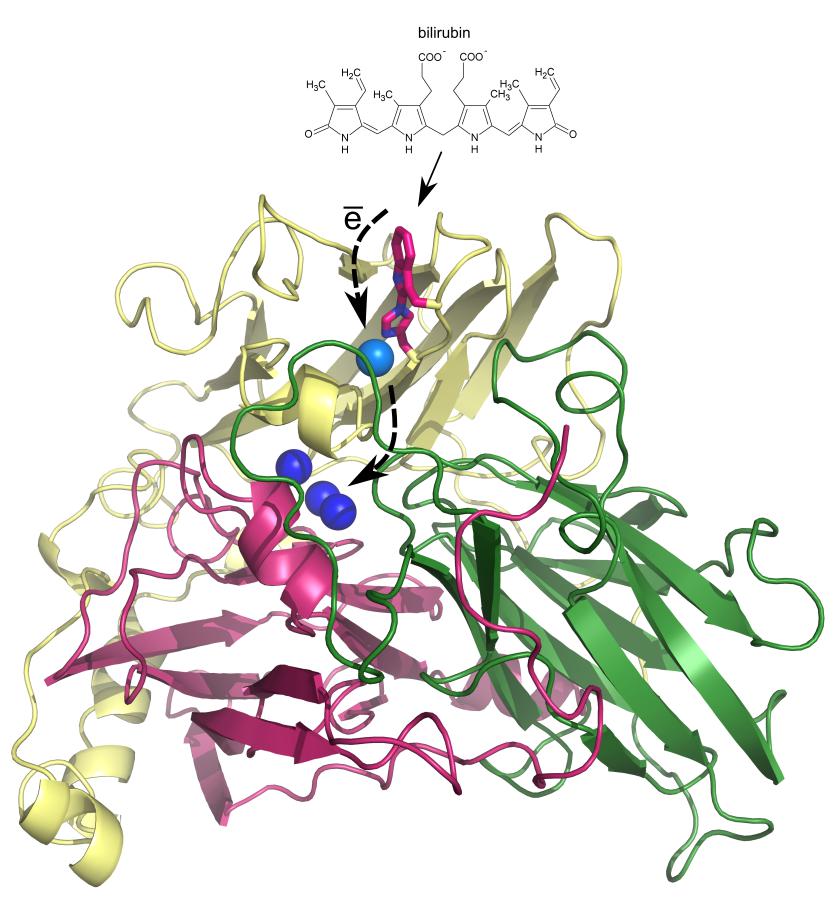
Protein bilirubin oxidase from parasitic fungus Myrothecium verrucaria is used in medicine for determination of bilirubin level. The protein reacts with bilirubin, taking away its electrons and transporting them via copper ions to oxygen molecule, which is subsequently transformed to two water molecules. Apart from bilirubin this enzyme reacts strongly also with other organic and inorganic molecules (substrates) and at various pH levels. Therefore the enzyme is also exploited in textile industry (textile decolorization, ecological degradation of textile waste) or in wood-processing industry (pulp decomposition). In recent years it has also been used in nanotechnologies, especially in the construction of bio-fuel cells and biosensors, where it is used as biocatalyst capable of efficient electron transfer. However, bilirubin oxidase is not only interesting from the point of view of applications but also for its actual structure and composition. Recently, a new type of chemical bond – unique in proteins – has been discovered in this enzyme. It is a bond between carbon of the side chain of amino acid tryptophan and nitrogen of amino acid histidine. Up to date, this bond seems to be present only in bilirubin oxidase.
Even if several teams around the world have studied bilirubin oxidase, it has not been clear exactly which parts of the enzyme interact with substrates, transfer electrons from substrate to copper ions and what is the function of the newly discovered bond. The Laboratory of Structure and Function of Biomolecules of the Institute of Biotechnology of the Czech Academy of Sciences led by Dr. Jan Dohnalek has focused on answering these questions. Extensive research resulted in determination of the substrate binding site, in the vicinity of the newly discovered bond. The results indicate that this bond plays a role in interactions between the enzyme and substrate, and shows selectivity towards various types of substances with different pH optima for reaction. The performed experiments proved that the removal of the bond led to enzyme deactivation with respect to specific compounds. Thanks to the identification of the substrate binding site also the possible route of electron transfer from substrate to copper ions could be discussed.
The new knowledge and results connected with the bond removal are of high significance, especially for development of new nanomaterials.
More information is available on:
https://www.nature.com/articles/s41598-019-50105-3